There are plenty of resources that say that no two snowflakes are alike. But where do we get this idea from? The universe is really old, with countless planets, surely two of them have been alike…
Wilson Bentley, an American meteorologist and photographer, worked with snowflakes for 50 years, without getting married, taking detailed photographs of snowflakes and recording their features. He called them ‘masterpieces of design’, but there doesn’t seem to be any design in any of them, but that does not make them any less amazing.

Every plate, every branch, every needle on a needle on a needle, all of those details, are what is called emergent properties. This is complexity that is based on very simple rules, and for snowflakes, those rules go back to the basic laws of physics. In the air, or in a liquid, water molecules are zipping around, bouncing off each other and every else trillions of times per second, and we have no way of knowing where they are or what direction they are facing at any moment. As we remove heat, it gets colder, and those water molecules start to slow down, eventually their atomic attraction, the actual hydrogen bonds between water molecules take over, and they settle into order. Sounds complicated, but we just call that ‘freezing’.

The structure of a snowflake can be found in just 6 water molecules. It is known that the angle between any two hydrogen is about 105 degrees. For some of those water molecules, the other hydrogen is behind them. Just like that, we’ve uncovered the six-fold symmetry of a snowflake crystal. That crystal starts as a tiny speck of dust, or pollen, which catches water vapor out of the air and eventually forms the simplest of snowflake shapes: tiny hexagons called diamond dust and then randomness kicks in. Till we get the intricate and delicate structures that we know and love.
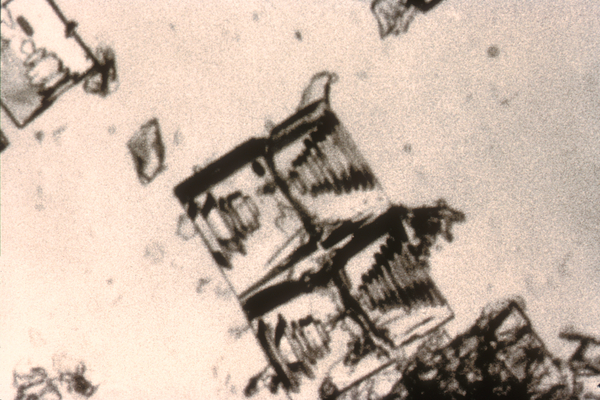
Depending on temperature and humidity, and a variety of factors that are not fully understood yet, those simple hexagons can give rise to seemingly infinite shapes. Each snowflake will travel through different air current and bump into different water molecules. But, in 1998, Nancy Knight claimed to find two identical snowflakes and they do look very much alike. But they cannot be alike and physics tells us why.
We know that water molecules are made of two hydrogen atoms and an oxygen atom, but not every hydrogen is created equally. If we go back to the Big Bang, we find out that out of every million or so hydrogen atoms created, a couple hundred of them, instead of just being a proton and an electron, are holding on to a neutron. This is the isotope of hydrogen called deuterium. In Earth’s water, even in you, about one in 3,000 molecules will be holding onto deuterium instead of hydrogen. Out of the millions of molecules that make up a snowflake, a lot of them will be holding onto deuterium. Even identical looking snowflakes are not the same.
Now you could love a snowflake just because it’s pretty, but it doesn’t take away from its beauty that it was sculpted by chance and physics. They’re ordered, but they’re created in disorder, every random branch re-tells their history, that singular journey that they took to get here, and most of all they’re fleeting and temporary.